- KAWAGUTI & PARTNERS Home>
- IP Laws & Practices>
- Overview of the Patent Term Extension in Japan
About Japanese Practices
Law & RulesOverview of the Patent Term Extension in Japan
4. What are the procedures to be followed?
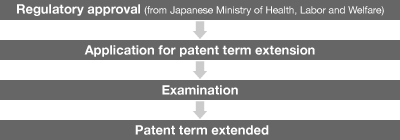
NOTE: After the issuance of the regulatory approval, the patent holder is required to file an application for patent term extension with the patent office within a certain period of time. Then, the application is examined by the examiner at the patent office.
5. Application for patent term extension
5-1. When to file it?(a) The application for patent term extension must be filed within 3 months* of the receipt of the regulatory approval;
(b) Before the original patent term expires; and
(c) When regulatory approval is not expected to be issued by the day before six months prior to the expiration of the original patent term, the applicant must submit a provisional application to previously notify the Patent Office as such.
NOTE: In regard to (c), the provisional application is just a notification and it will NOT extend the due period in (a) and (b) in any way.
5-2. Who will file it?(a) The application must be filed in the name of patent holder; and
(b) If there are any co-holders, all of them must be included.
NOTE: The application must be filed in the name of patent holder. If there are any co-holders, all of them must be included. If not included, the application is to be rejected.
5-3. How to identify the relationship between the patentee and the recipient of regulatory approval?Where the recipient of the regulatory approval is not the patentee, the recipient must be registered* as a licensee** with the Patent Office.
*Registration procedure can be initiated after filing the application of patent term extension but the registration must be completed before the end of the examination.
**Exclusive or non-exclusive licensee.
NOTE: The certificate of extension of patent term will be issued on the application only if the patent owner or its exclusive or non-exclusive licensee is the recipient of the regulatory approval. Therefore, if the recipient of the regulatory approval is not the patentee, the recipient must be registered as a licensee in the Patent Office.
In this relation, an exclusive license is only effective if the licensee is registered with the patent office. Generally, a non-exclusive license is still effective even if the licensee is not registered with the patent office. However, to enjoy the patent term extension, the non-exclusive licensee also needs to be registered with the patent office so that the relationship between the patent holder and the licensee can be easily recognized.
In this regard, the registration procedure can be initiated after the application of the patent term extension but the registration must be completed before the decision of patent term extension issues.
5-4. What are the necessary documents?(a) a copy of the regulatory approval from the Japanese Ministry of Health (Japanese FDA);
(b) a copy of the IND*; and
(c) a signed license agreement (if appropriate).
*Including a copy of IND filed with the foreign authority in relation to clinical trials in such country: If the clinical trial results in the foreign country are to be considered by the Japanese FDA, the day when the IND was filed with the foreign authority is counted as the starting point of the regulatory review period in Japan.
NOTE: An application for patent term extension must be accompanied by the following documents; (a) a copy of the regulatory approval from the Japanese Ministry of Health (Japanese FDA); (b) a copy of the IND; and (c) a signed license agreement (if appropriate).
In this connection, a copy of the IND filed with the foreign authority in relation to clinical trials in such country may be included. That is, if the clinical trial results in the foreign country are to be considered by the Japanese FDA, the day when the IND was filed with the foreign authority is deemed as the starting point of the regulatory review period in Japan.