- KAWAGUTI & PARTNERS Home>
- News Letter>
- October, 2015
K&P’sIntellectual Property High Court Decision Report
October, 2015
Updated 1 APR 2016
1. To What Degree is Disclosure of Specification Required for Satisfying Enablement Requirement for Use Claims of Combined Medicine?Chimerix, Inc. v. Commissioner of JPO, Case No. 2015 (Gyo-Ke) 10021 (Decision rendered on October 13, 2015)
The Applicant, Chimerix, filed a patent application relating to a pharmaceutical composition for the treatment of viral infections in 2006, but the application was finally rejected in 2014 by the Appeal Board of the JPO on the grounds of not satisfying the enablement requirement and the support requirement. Against the JPO's decision, Chimerix filed a cancellation action with the IPHC in 2015.
Claim 1 of the Chimerix's patent application at issue claims as follows:
1. A pharmaceutical composition for the treatment of a viral infection, the composition comprising a pharmacologically effective amount of compound having the following structure:
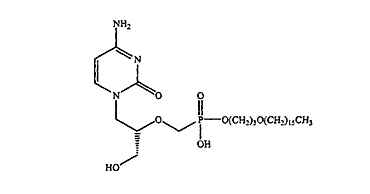
(HDP-CDV)
or a pharmaceutically acceptable salt thereof, and at least one immunosuppressant,
wherein the viral infection is … … .
The main issue in this case lied in to what degree the disclosure of the specification is required for satisfying the enablement requirement for use claims of a combined medicine. The IPHC answered to the issue as follows:
First, having reviewed the specification of the patent application at issue, the IPHC found that the specification suggested that when HDP-CDV, which was a known compound, was used in combination with an immunosuppressant, the immunosuppressant enhanced the biological availability of HDP-CDV. However, having reviewed the prior art references of the patent application at issue in detail, the IPHC also found that it had been common technical knowledge at the time of the filing date of the patent application at issue that the administration of an immunosuppressant was prone to develop virus infections.
On the basis of the above findings, the IPHC concluded (i) that even though those skilled in the art could have understood that the administration of an immunosuppressant in combination with HDP-CDV enhanced the bioavailability of HDP-CDV, there were concerns that the administration of immunosuppressant might have aggravated infections due to immune suppression brought by using an immunosuppressant in light of the foregoing common technical knowledge, thus (ii) that it was not evident whether a treatment effect could be further improved by using HDP-CDV in combination with an immunosuppressant compared to the case using HDP-CDV independently, and therefore the patent application at issue did not satisfy the enablement requirement.
Conclusively, the IPHC dismissed the Chimerix's appeal, and maintained the JPO's decision.
An appeal to the Supreme Court was NOT filed against this decision, and thus the decision is final and binding.
K&P’s CommentsUnder the Japanese patent practice, specific pharmacological data and the like are generally required to be disclosed in the specification as filed for satisfying the enablement requirement for medical use claims. In the specification of the patent application at issue, there are no specific data for the claimed combined medicine, although some data showing that HDP-CDV is excellent in antivirus activity are disclosed therein. Accordingly, this case teaches that it would be essential to demonstrate in the specification as filed that the claimed combined medicine itself shows an advantageous treatment effect for satisfying the enablement requirement for the combined medicine, in particular, when there is any negative common technical knowledge as appeared in this case.
In October 2015, the IPHC handed down 20 decisions including the above case on patent and utility model, and overturned a previous decision in 1 case.
In October 2015, the IPHC handed down 4 decisions on trademark, and maintained all of the previous decisions.



