- KAWAGUTI & PARTNERS Home>
- News Letter>
- July, 2017
K&P’sIntellectual Property High Court Decision Report in 2017
July, 2017
Updated 4 JAN 2018
1.When Data include an Erroneous Description, Are the Enablement and Support Requirements Violated by the Data?
X (Individual) v. Seattle Genetics, Inc., Case No. 2016 (Gyo-Ke) 10146 (Decision rendered on July 12, 2017)
The Patentee, Seattle Genetics (SG), obtained a patent relating to a method of making a modified antibody in 2014. Against the SG’s patent, X, an individual, filed an invalidation trial with the JPO in 2015. The JPO dismissed the X’s demand in 2016. X filed an appeal against the JPO's decision to the IPHC in 2016.
Claim 1 of the SG’s patent at issue claims as follows:
A method of making a modified antibody or antibody derivative having reduced core fucosylation, comprising:
culturing a host cell in a culture medium comprising an effective amount of a fucose analog under suitable growth conditions, ..., and
isolating the antibody or antibody derivative from the cells,
wherein the fucose analog is selected from the group consisting of one of the following formulae (III) or (IV):
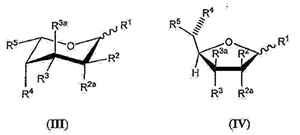
or a biologically acceptable salt or solvate thereof, wherein …; and
wherein the antibody or antibody derivative has reduced core fucosylation compared to the antibody or antibody derivative from the host cell cultured in the absence of the fucose analog; or ….
One of the main issues in this case relates to whether the support and enablement requirements are satisfied when the experimental data erroneously show that one compound, which falls into the claimed scope, exerts no beneficial effects. The IPHC answered to the issue as follows:
Regarding the enablement and support requirements, X, the Plaintiff, argued as follows:
(1) According to Table 1 in the specification, when another pivalate ester group is attached to alkynyl fucose di(trimethylacetate) which shows ">80% of inhibitory activity at 50 μM", the inhibitory activity is reduced to "~0%" (cf. at 50 μM of alkynyl fucose 1,2,3-tri(trimethylacetate)). Thus, it is common technical knowledge that even a slight change in the chemical structure dramatically affects the activity. Accordingly, not all of the fucose analogs recited in claim 1 can be used for production of antibodies with reduced core fucosylation [Violation of Enablement Requirement].
(2) Although the decision of the invalidation trial regarded the numerical value of alkynyl fucose 1,2,3-tri(trimethylacetate) as unnatural, the decision took into account the raw data that SG, the Patentee, submitted after submitting the Written Reply during the invalidation trial procedure. Thus, the decision of the invalidation trial is based on the raw data that was not available to those skilled in the art as of the filing date [Violation of Enablement Requirement].
(3) Since the specification discloses that the activity of alkynyl fucose 1,2,3-tri(trimethylacetate) in reducing core fucosylation is "~0%", the claimed invention is definitely beyond the extent of the disclosure in the description. There is no rational reason that those skilled in the art would recognize that the above numerical value is erroneously described, and that the fucose analog can naturally solve the problem to be solved by the invention [Violation of Support Requirement].
The IPHC decided that the enablement and support requirements are satisfied. The reasons are as follows:
The specification has general descriptions about antibodies or antibody derivatives having the Fc domain having the complex N-glycoside-linked sugar chain, the host cells expressing the antibody or antibody derivative, the fucose analog to be added to the culture medium, the method for culturing the cells, and the purification of the antibodies or antibody derivatives. Experimental examples regarding synthesis of fucose analogs with various substituents, production of antibodies, core fucosylation of the antibodies, and analysis of biological activity are also disclosed. Thus, even though the biological activity varies depending on the chemical structure of the fucose analogs, a considerable number of fucose analogs were examined in the specification, and the specification sufficiently confirms that the fucose analogs can inhibit core fucosylation. There is no basis for denying that other fucose analogs can also inhibit the core fucosylation.
The IPHC did not adopt the X (Plaintiff)'s arguments for the following reasons:
Regarding (1), those skilled in the art would recognize that the numerical value of alkynyl fucose 1,2,3-tri(trimethylacetate) is unnatural, and measure the inhibitory activity of the fucose analog according to the method disclosed in the Examples of the specification. Those skilled in the art would find with no particular technical difficulty that the numerical value of the fucose analog shown in Table 1 is erroneous.
Regarding (2) and (3), those skilled in the art would presume that the numerical value of alkynyl fucose 1,2,3-tri(trimethylacetate) is an erroneous description, which can be easily confirmed based on the Examples of the specification. Thus, in this case, the data supplemented after the filing date can be taken into account in considering the enablement and support requirements. SG, the Patentee, noticed that one of the various experimental data is erroneous, and submitted the post-filing analyzed data which show the results within the disclosures of the specification, so as to offer the explanation. This is a different case from those in which, for example, an applicant subsequently submits a post-filing experimental data which should have been available prior to the filing date.
Conclusively, the IPHC dismissed the X’s appeal and upheld the JPO’s decision.
An appeal to the Supreme Court was filed against this decision, and thus the decision is NOT final and binding.
K&P’s Comments According to this decision, even though the experimental data which fall into the claimed scope include an erroneous description and thereby exert no beneficial effect, the enablement and support requirements may be satisfied under the conditions: i) that, in light of the description of the specification and the common technical knowledge, those skilled in the art would recognize that the description is unnatural (erroneous); and ii) that those skilled in the art would confirm with no particular technical difficulty that the description is erroneous, based on the specification.
In this case, it is important that a considerable number of the experiments (about 40 analogs) are disclosed in the specification. It would be preferable to disclose as much experimental data as possible in the specification.
2. Whether Correction of Concentration of Component on the basis of Numerical Value Indicated in Working Example should be Accepted when Value did not Directly Correspond to Claimed Concentration, but was Technically Related Thereto?
San-Ei Gen F.F.I., Inc. v. JK Sucralose Inc., Case No. 2016 (Gyo-Ke) 10157 (Decision rendered on July 19, 2017)
The Patentee, San-Ei Gen F.F.I. (SEG), obtained a patent relating to a method for masking acidity in 2007. Against the SEG’s patent, JK Sucralose (JKS) filed an invalidation trial with the JPO in 2014. During the trial proceedings, SEG demanded a correction of claims, but the JPO dismissed the correction, and rendered a decision of invalidation in 2016. SEG filed an appeal against the JPO's decision to the IPHC in 2016.
Claim 1 of the SEG’s patent before the correction at issue claims as follows:
A method for masking acidity, comprising adding sucralose to a product containing fermented vinegar and/or apple-cider vinegar or a product containing coffee extract in an amount of 0.000013 to 0.0042% by weight of the product.
Claim 1 of the SEG’s patent after the correction at issue claims as follows:
A method for making acidity of a product containing fermented vinegar and/or apple-cider vinegar, comprising adding sucralose to the product in an amount of 0.0028 to 0.0042% by weight of the product.
One of the main issues in this case related to whether the correction of the concentration of a component on the basis of a numerical value indicated in a working example should be accepted when the value did not directly correspond to the claimed concentration, but was technically related thereto. The IPHC answered to the issue as follows:
During the proceeding of the invalidation trial, SEG filed a correction of Claim 1 on the basis of the disclosure of Example 2. Specifically, Example 2 demonstrated as below:
Water was added to 15 parts of fermented vinegar (acidity: 10%), 6.5 parts of salt, … , 0.0028 part of sucralose, … to be 100 parts, to which appropriate amount of laurel, cashier and hot pepper were added to prepare a liquid preparation. The obtained liquid preparation and desalted cucumbers were combined in a proportion of 4 to 6 and were bottled to make pickles having milder acidity compared to pickles made with no sucralose added.
In the JPO’s decision, the JPO found that “0.0028% by weight” was the concentration of sucralose in the liquid preparation, and not the concentration of sucralose in the pickles on which the masking effect on acidity was confirmed, and therefore the concentration of sucralose in the pickles was unknown; and decided that it was not led on the basis of Example 2 that the lower limit of the sucralose concentration was “0.0028% by weight”, and accordingly that the correction introduced a new technical matter.
However, the IPHC overturned the above JPO’s decision as stated below.
(1) As the JPO found, “0.0028% by weight” was the concentration of sucralose in the liquid preparation, and not the concentration of sucralose in the pickles on which the masking effect on acidity was confirmed.
(2) However, it was technically clear that the concentration of sucralose in the pickles was lower than 0.0028% by weight due to water derived from cucumbers.
(3) Once the masking effect on acidity was confirmed at a concentration of sucralose lower than 0.0028% by weight in the pickles, it was clear for those skilled in the art on the basis of the disclosure of the specification of the patent at issue and common technical knowledge that the masking effect on acidity was brought about at the concentration of sucralose of 0.0028% by weight in the pickles.
(4) Accordingly, it was acknowledged that the specification disclosed that the masking effect on acidity was brought about at the lower limit of “0.0028% by weight” in the product; and thus the correction was made within the disclosure of the specification, and introduced no new technical matter.
Conclusively, the IPHC upheld the SEG’s appeal, and cancelled the JPO’s decision.
An appeal to the Supreme Court was NOT filed against this decision, and thus the decision is final and binding.
K&P’s Comments As seen from the above decision, the IPHC accepted the correction to specify the lower limit of the concentration of the product as “0.0028% by weight” on the grounds that the correction did not introduce any new technical matter in view of the disclosure of the specification of the patent at issue and common technical knowledge, although the figure “0.0028% by weight” indicated in Example 2 was not the concentration of the product, but that of the liquid preparation. This decision teaches a new possible approach for correction.
In July 2017, the IPHC handed down 15 decisions including the above two cases on patent, and overturned the previous decisions in 5 cases.
In July 2017, the IPHC handed down 7 decisions on trademark, and maintained all of the previous decisions.
In July 2017, the IPHC handed down no decision on industrial design.



