- KAWAGUTI & PARTNERS Home>
- IP Laws & Practices>
- Overview of the Patent Term Extension in Japan
About Japanese Practices
Law & RulesOverview of the Patent Term Extension in Japan
7. Scope of protectionScope of the protection of the patent right during the extended patent term is determined in relation to the regulatory approval on which the extended patent term relies:
NOTE: As in the above, in Japan, a patent may be extended more than once based on different regulatory review periods. Moreover, more than one patent may be extended even based on a single regulatory approval.
Examples
Case I: Regulatory approval for Compound A and Viral infections treatment
| Claims | Scope of protection | |||
| Patent | Compound | Use | Active ingredient | Indication |
|---|---|---|---|---|
| Product | A,B and C | - | A | Anti-viral |
| Use | A,B and C | Anti-viral | A | Anti-viral |
| Process | A,B and C | - | A | Anti-viral |
Case I: Regulatory approval for Compound A and Viral infections treatment
| Claims | Scope of protection | |||
| Patent | Compound | Use | Active ingredient | Indication |
|---|---|---|---|---|
| Product | A,B and C | - | B | Anti-cancer |
| Use | A,B and C | Anti-viral | - | - |
| Process | A,B and C | - | B | Anti-cancer |
NOTE: The above shows the scope of protection provided by the extended patent term in relation to the regulatory approval.
In the first case, the regulatory approval is issued for a product containing Compound A (active ingredient) for use in viral infections treatment (indication). Then, the scope of protection provided by the extended patent is limited to the working of the patented invention in relation to Compound A and viral infections treatment. In other words, the product patent is only enforceable against infringers who prepare, use, assign and/or import the product containing Compound A for the ultimate purpose of viral infection treatment. The process patent is also enforceable against infringers who carry out the patented process to prepare Compound A that is used to manufacture a drug product for use in viral infections treatment.
Calculation of the extended patent term
Case: Product patent broadly covers Compounds A, B and C; Use is not specified in the claims. The INDs were filed after the patent grant.
1. First regulatory approval
Regulatory review period: 3 years
Active ingredient: Compound A
Indication: Viral infections treatment
2. Second regulatory approval
Regulatory review period: 4 years
Active ingredient: Compound B
Indication: Cancer treatment
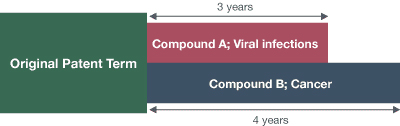
NOTE: The above illustrates the relationship between the patent term extended based on the first and second regulatory approvals and the scope of protection. The patentee may enjoy the extended patent term of 3 years with regard to Compound A and viral infections treatment and the term of 4 years with regard to Compound B and cancer treatment. In other words, there may be many different rights after the original patent term expires.
8. Our RemarksIn view of the above, in order to enjoy the maximum protection in terms of the extended patent term, enhancing the prosecution of the patent application is beneficial. That is, the regulatory review period serving as a basis for an extended patent term may be reduced by the delay of the patent prosecution. To this end, requesting an accelerated examination is worth considering.
In terms of the scope of protection during the extended patent term, considering which patent or patents are likely to get extended relative to given regulatory approval is important.
Kawaguti & Partners