| 2012.10.01 |
K&P's Intellectual Property High Court Decision Report in April, 2012 |
 |
1. Novelty of Combination Drug
Sawai Pharmaceutical Co. Ltd. v. Takeda Pharmaceutical Co. Ltd., Case No. 2011 (Gyo-Ke) 10148 (Decision rendered on April 11, 2012)
The Patentee, Takeda, obtained a patent relating to a combination drug for treating diabetes in 2001, against which Sawai filed an invalidation trial before the JPO in 2010. The JPO dismissed Sawai's demand in 2011, followed by Sawai's appeal against the JPO's decision to the IPHC in 2011.
The patent in issue claims as follow:
"A drug for prophylaxis or treatment of diabetes or diabetic complications, comprising (1) pioglitazone or a pharmaceutically acceptable salt thereof in combination with (2) an α-glucosidaze inhibitor selected from acarbose, voglibose and miglitol".
The main issue in this case was novelty of the combination drug, on which the IPHC decided as below.
Firstly, the IPHC found that it was a common technical knowledge at the time of the priority date of the present patent that insulin-susceptible enhancers which include pioglitazone, and α-glucosidaze inhibitors which include acarbose, voglibose and miglitol, had different mechanism of action from each other relating to the decrease of the level of blood glucose. Furthermore, the IPHC found that in the case where drugs having different mechanisms of action from each other were used in combination, it was unlikely in general that these drugs were antagonistic to each other, and thus it was considered that they would exert their action through the respective mechanisms and that the respective effects would be individually produced.
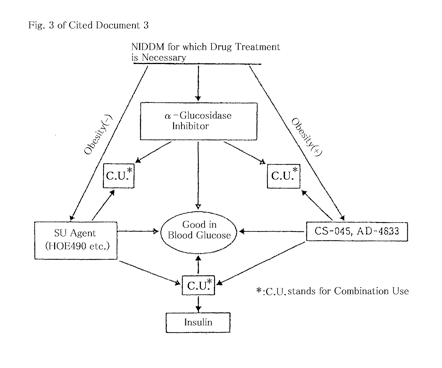
On the basis of the above findings and the disclosure of cited document 4, the IPHC decided that the technical concept of the administration of an insulin-susceptible enhancer and an α-glucosidase inhibitor in combination to diabetic patients had been publicly known at the time of the priority date of the present patent, and thus Fig. 3 of cited document 3 (see attached diagram) was deemed to disclose using pioglitazone (AD-4833) and α-glucosidase inhibitors, such as acarbose, voglibose and miglitol, in combination.
Moreover, the IPHC also decided that those skilled in the art at the time of the priority date of the present patent naturally could have envisaged that the use of drugs having different mechanisms of action with each other for treating diabetes in combination would bring about at least a so-called additive effect even if they could not have envisaged that the use would bring about a so-called synergistic effect in view of Fig. 3. Nevertheless, the disclosure of the specification of the present patent demonstrated only the additive effect and it did not sufficiently support the synergistic effect.
Conclusively, the IPHC upheld Sawai's appeal and cancelled the JPO's decision.
An appeal to the Supreme Court was filed against this decision, and thus the decision has not been conclusively affirmed.
|
 |
K&P's Comments
This decision would teach that it is quite important to show a synergistic effect over an additive effect in the text of specification, in particular in the working examples, as filed in order to obtain a patent relating to a combination drug having different mechanisms of action from each other.
|
 |
2. Inventive Step of Claimed Property of Resin Pipe
Kubota Corp. et al. v. Sekisui Chemical Co. Ltd., Case No. 2011 (Gyo-Ke) 10186 (Decision rendered on April 11, 2012)
The Patentee, Sekisui, obtained a patent relating to a hard vinyl chloride resin pipe in 2008, against which Kubota and Kubota-C.I. (hereinafter collectively referred to as "Kubota") filed an invalidation trial before the JPO in 2010. The JPO dismissed Kubota's demand in 2011, followed by Kubota's appeal against the JPO's decision to the IPHC in 2011.
The patent in issue claims as follow:
"A hard vinyl chloride resin pipe to which an organic black pigment is added as a pigment, wherein Δσ is 2.94 MPa or less, Δσ being the difference between the maximum value and the minimum value of the circumferential stress σ after being exposed under the environment of the solar radiation of 3,500 kcal/m2�day or more for 20 days ".
The main issue in this case was inventive step of the claimed property "Δσ", on which the IPHC decided as below.
Firstly, the IPHC found that the prior art article 1 had been exposed under the same environment as claimed from January 15, 2010 to March 11, 2010, and Δσ thereof was less than 2.94 MPa.
In this connection, the JPO had decided that it was not able to admit that article 1 satisfied the value of the claimed Δσ before the filing date of the present patent since it was a common technical knowledge that residual stress generally varied with time and Kubota did not show that Δσ did not change with time.
However, the IPHC found that it was appropriate to infer that article 1 satisfied the value of the claimed Δσ before the filing date of the present patent since article 1 had been stored indoors without severe solar radiation etc. and it was unlikely that the value of Δσ decreased due to stress relaxation with time and improper storage although it might have increased.
On the basis of the above findings, the IPHC decided that the pipe which satisfied the value of the claimed Δσ had been publicly used at the time of the filing date of the present patent and the claimed numerical limitation of "2.94 MPa or less" was just to specify the allowable curvature degree by the value of σ, in which no special technical meaning was found, and therefore it was inappropriate to determine novelty and inventive step on the basis of the claimed Δσ. Namely, the IPHC did not find the claimed Δσ as a different feature from the main prior art document.
Finally, the IPHC concluded that the JPO's decision that the claimed Δσ was not easily conceivable was based on a misunderstanding of the fact premise, and thus the JPO's decision could not be accepted. The IPHC cancelled the JPO's decision.
An appeal to the Supreme Court was filed against this decision, and thus the decision has not been conclusively affirmed.
|
K&P's Comments
This decision would teach that even the claimed prima-facie novel property may not be considered as a technical feature distinguishable from the prior art in the case where such a property is a commonplace property of conventional articles.
In April 2012, the IPHC handed down 14 decisions including the above 2 cases on patent, and overturned the previous decisions in 4 cases. |
 |
| Nakamura’s press conference is expected on January 31, 2009. |
|

