- KAWAGUTI & PARTNERS Home>
- News Letter>
- January, 2015
K&P’sIntellectual Property High Court Decision Report
January, 2015
Updated 1 JUL 2015
1. How Should Identicalness of Invention Relating to Crystalline Polymorph be Examined? F. Hoffmann-La Roche AG v. Commissioner of JPO, Case No. 2013 (Gyo-Ke) 10285 (Decision rendered on January 22, 2015)
The Applicant, Roche, filed a patent application relating to an ibandronate polymorph form A in 2006, but the application was finally rejected on the grounds of being identical to an invention disclosed in a prior application by the Appeal Board of the JPO in 2013. Against the JPO’s decision, Roche filed a cancellation action before the IPHC in 2013.
Claim 1 of the patent application at issue claims as follows:
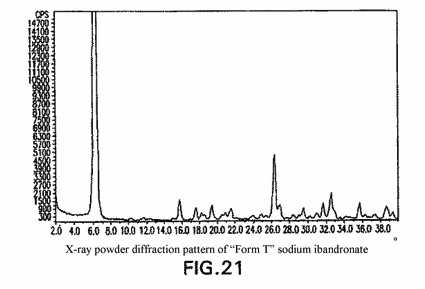
A crystalline polymorph of 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid monosodium salt monohydrate (ibandronate) which is characterized by an X-ray powder diffraction pattern having characteristic peaks expressed in angle 2-θ at 10.2°, 11.5°, 15.7°, 19.4° and 26.3°.
The main issue in this case lied in how the identicalness of the invention relating to a crystalline polymorph should be examined. The IPHC answered to the issue as follows:
In the JPO’s decision, the JPO found that the prima-facie difference between the invention of the patent application at issue and that disclosed in prior application (p.a.) 1 lied only in that the former specified “10.2°” and “11.5°” as the characteristic peaks, while the latter did not specify them. Regarding the difference, the JPO concluded that the characteristic peaks of “10.2°” and “11.5°” were able to be detected in Fig. 21 of p.a. 1 (refer to the Figure below) and thus the difference was not substantial.
However, having reviewed p.a. 1 in detail, the IPHC first found that p.a. 1 did not mention whether “Form T” disclosed in p.a. 1 was a solvate, and, if it be so, what type of form the solvate had, though p.a. 1 disclosed the data of the weight loss determined by TGA for 21 kinds of solid crystalline forms of sodium ibandronate. On the basis of the above finding, the IPHC concluded (i) that those skilled in the art must have understood that it could not be ascertained whether “Form T” was a solvate from p.a. 1, (ii) that it could not be anticipated that the water related to the weight loss was either adhesive moisture or crystallization water only from the preparation method of “Form T” and the results of the analysis by TGA disclosed in p.a. 1, and thus (iii) that it could not be acknowledged that “Form T” was a monohydrate, and therefore the JPO’s finding that “Form T” disclosed in p.a. 1 was a monohydrate was incorrect.
Further, having reviewed Fig. 21 of p.a. 1, the IPHC found that it could not be acknowledged that the characteristic peaks were detected at “10.2°” and “11.5°” in Fig. 21, since the peaks corresponding to “10.2°” and “11.5°” in Fig. 21 were extremely weak and faint even if the peaks could be perceived at those angles, and concluded that the above prima-facie difference was substantial.
Conclusively, the IPHC upheld the Roche's appeal, and cancelled the JPO's decision.
An appeal to the Supreme Court was NOT filed against this decision, and thus the decision is final and binding.
K&P’s Comments Patent applications claiming a specific crystalline polymorph of drug compounds are often filed in Japan for substantially extending the patent term of the compounds. This decision provides an example of how to examine the identicalness of an invention relating to a crystalline polymorph.
In January 2015, the IPHC handed down 17 decisions including the above case on patent, and overturned previous decisions in 5 cases.
In January 2015, the IPHC handed down 6 decisions on trademark, and overturned previous decisions in 1 case.



