- KAWAGUTI & PARTNERS Home>
- News Letter>
- 2016 <Special News Flash>
K&P’sIntellectual Property High Court Decision Report in 2016
2016<Special News Flash>
Updated 1 APR 2016
IPHC Finds Infringement under Doctrine of Equivalents (DOE) on Patent relating to Process for Preparing Compound against Generic Drug Makers
DKSH Japan Co., Ltd., Iwaki Seiyaku Co., Ltd., Takata Pharmaceutical Co., Ltd. and Pola Pharma Inc. v. Chugai Pharmaceutical Co., Ltd., Case No. 2015 (Ne) 10014 (Decisions rendered on March 25, 2016)
The Grand Panel1) of the IPHC (Chief Judge, Ryuichi Shitara) decided that the above 4 Appellants’ (generic drug manufactures’) accused process for preparing a compound, which is used as a drug for treating psoriasis, constitutes an infringement under the DOE, which is the first case where the IPHC finds an infringement under the DOE in the pharmaceutical field.
Corrected Claim 13 of the Chugai’s patent at issue claims as follows:
Claim 13. A process for preparing a compound having the following formula:
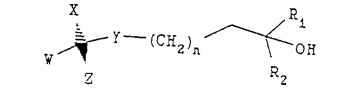
wherein n is 1, R1 and R2 are methyl, each of W and X is independently hydrogen or methyl, Y is O, and Z is a steroid ring structure of the formula: <snip>
or a vitamin D structure of the formula:
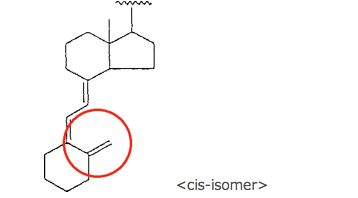
wherein … …
which comprises:
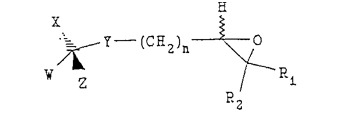
(a) reacting a compound having the following structure:

wherein W, X, Y and Z are as defined above,
in the presence of a base, with a compound having the following structure:
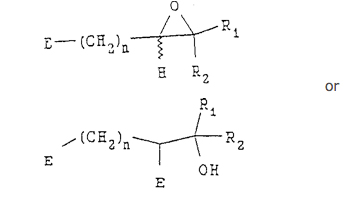
wherein n, Rl and R2 are as defined above, and E is an eliminating group,
to produce an epoxide compound having the following structure:
(b) treating the epoxide compound with a reducing agent to produce the compound; and
(c) recovering the compound so produced.
In this infringement case, there was a difference between the corrected claim 13 and the accused process in that the former defined the carbon skeleton of the starting material and the intermediate material as the cis-structure of vitamin D, while the latter used the starting and the intermediate material having the trans-structure thereof. One of the main issues in this case lied in how “the essential elements” in the 1st factor for the DOE should be construed. The IPHC answered to the issue as follows:
In 1998, the Supreme Court ruled 5 factors for finding an infringement under the DOE, and the 1st one was “the different elements between the patented invention and the accused product or process are not the essential elements in the patented invention” [Supreme Court Decision of February 24, 1998, Case No. 1994 (O) 1083].
Regarding the 1st factor, the Grand Panel first generally ruled (1) that “the essential elements” of the patented invention in the 1st factor for the DOE should be construed as the characteristic feature constituting a particular technical concept which could not have been found in the prior art techniques, and (2) that “the essential elements” should be found first by perceiving the problem to be solved by the patented invention, the means for solving the problem, and the effect of the patented invention on the basis of the claims and the specification of the patented invention, and then by determining what was the characteristic feature constituting a particular technical concept which could not have been found in the prior art techniques. Namely, “the essential elements” should be found by comparing with the prior art technique in the specification of the patent since the substantial value of the patented invention should be determined depending on the degree of contribution of the patented invention compared to the prior art techniques. Therefore, “the essential elements” should be recognized as broader concept if the degree of the contribution was high, while “the essential elements” should be recognized as about the same as the literal scope of the claim if the degree of the contribution was not so high.
Further, the Grand Panel also ruled that it should not be construed that each of the claimed elements should be first divided into “the essential elements” and non-essential elements, and then no DOE was found at all for the elements corresponding to “the essential elements”.
On the basis of the above ruling, the Grand Panel decided (1) that the degree of the contribution of the corrected invention was high since the corrected invention enabled to prepare the objective substance through a new preparation route which could not have been found, (2) that “the essential elements” of the corrected invention lied in to find that an side chain having an epoxy group through an ether bond could be introduced by one step by reacting an alcoholic compound including the structure of vitamin D with an epoxy hydrocarbon compound having an eliminating group at the end of the compound, and then to enable to introduce the side chain of maxacalcitol to the alcoholic compound having the structure of vitamin D by a new route of (a) going through the intermediate compound having the structure of vitamin D into which the side chain having an epoxy group through an ether bond was introduced, and then (b) ring-opening the epoxy group in the side chain of the intermediate; and (3) that “the essential elements” did not include that the structure of vitamin D was defined as cis-isomer since there was no difference between cis-isomer and trans-isomer in that (i) the starting material was reacted with an epoxy hydrocarbon compound having an eliminating group at the end of the compound to obtain an intermediate into which a side chain having an epoxy group through an ether bond was introduced, and then (ii) the side chain of maxacalcitol was able to be introduced by open-ringing the epoxy group in the side chain.
Conclusively, the IPHC dismissed the generic drug manufactures' appeal and upheld the decision rendered by the 1st instance, i.e. the Tokyo District Court.
The lost generic drug manufactures are entitled to bring the case to the Supreme Court within 2 weeks after the mailing date of the decision, and thus the decision is NOT final and binding to date.
1) Grand Panel: Grand Panel is convened to hear cases where it is necessary to virtually unify court decisions, or cases involving important issues, which is somewhat similar to en banc in CAFC of the U.S.A. Since the founding of the IPHC in 2005, 10 cases including the above case have been heard.
K&P’s CommentsThis case has been watched with interest since the 1st instance, the Tokyo District Court first found an infringement under the DOE on a patent relating to a process for preparing a compound in the pharmaceutical field in Japan. As shown above, the Grand Panel clearly ruled that the scope covered by the DOE can vary depending on the degree of the contribution of the patented invention compared to the prior art techniques, and upheld the district court's decision. This decision could make a great impact on the pharmaceutical field where originator pharmaceutical companies and generic drug manufacturers have been competing fiercely.



