- KAWAGUTI & PARTNERS Home>
- News Letter>
- May, 2017
K&P’sIntellectual Property High Court Decision Report in 2017
May, 2017
Updated 1 NOV 2017
1. To What Extent should Correction of Error in Description After Grant be Accepted?
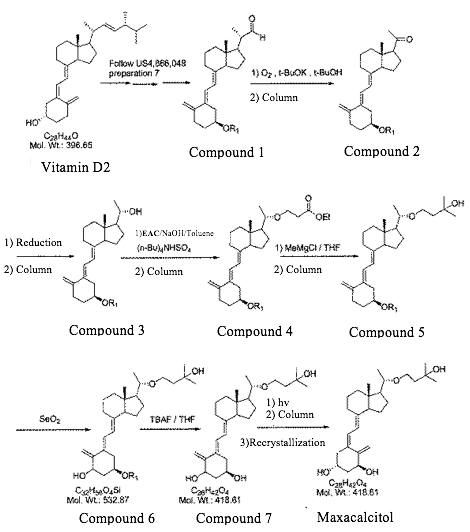
Formosa Laboratories Inc. v. Commissioner of JPO, Case No. 2016 (Gyo-Ke) 10154 (Decision rendered on May 30, 2017)
The Patentee, Formosa, obtained a patent relating to a maxacalcitol intermediate and a process for producing the same in 2014. Thereafter, Formosa filed a correction trial with the JPO in 2015.
In the trial, Formosa demanded to correct the description “EAC (ethyl acetate, 804 ml, 7.28 mol)” in the Examples (para. 0034) of the specification to “EAC (ethyl acrylate, 804 ml, 7.28 mol)”. The JPO dismissed the Formosa’s demand in 2016 on the grounds that the description that the Patentee demanded the correction was not considered as a clear error in the description, followed by the Formosa's appeal against the JPO's decision to the IPHC in 2016.
The main issue in this case related to what extent a correction of error in the description after the grant should be accepted. The IPHC answered to the issue as follows:
First, the IPHC generally ruled that for accepting correction of “an error in the description” after the grant, it was necessary (i) that it was clear from the disclosure of the specification and common technical knowledge that the description before the correction included an error and a correction made to the description was correct and (ii) that it was natural for those skilled in the art to notice that an error existed and deem that the error should have read as the corrected description.
On the basis of the above ruling, the IPHC examined the demanded correction at issue step by step as follows:
(1) Whether those skilled in the art could notice that there was any error in the description of the specification
Para. 0034 of the specification at issue demonstrated the process of obtaining Compound (4) from Compound (3) under the conditions where “EAC” was added (refer to the synthesis scheme below). In this regard, para. 0034 specifically stated that EAC (ethyl acetate, 804 ml, 7.28 mol) was added to and reacted with Compound (3) to obtain Compound (4).
However, those skilled in the art could notice that the side chain of -OCH2CH2COOEt in Compound (4) could not be obtained (since one carbon atom is lacking) if -OH group of Compound (3) nucleophilically attacked the carbon atom of ethyl acetate (CH3COOEt). Accordingly, those skilled in the art could notice that there was an error in any of Compound (3), EAC (ethyl acetate) and Compound (4).
(2) Whether those skilled in the art could understand that the error was “EAC (ethyl acetate)”
Compound (3) was the same compound as defined in the claims and no technical contradiction was found in obtaining Compound (3) from Compound (2) by referring to the descriptions of paras. 0030 to 0033 of the specification. Accordingly, those skilled in the art found no reason that the structure of Compound (3), in particular the structure where -OH group was bonded to the carbon atom at position 5, was incorrect. Further, those skilled in the art could understand (i) that the structure of Compound (5) was correct since the side chain of Compound (5) was the same as that of the final product, maxacalcitol, and (ii) that comparing the structure of the side chain of Compound (5) and that of Compound (4) and considering the reaction mechanism, there was no error on the structure of the side chain of Compound (4). On the other hand, those skilled in the art could easily confirm that 804 ml of ethyl acetate corresponds to 8.21 mol thereof, which was contradictory to the description of specification. Accordingly, those skilled in the art could understand that the chemical structures of Compounds (3) and (4) were correct, while “ethyl acetate” was incorrect.
(3) Whether those skilled in the art who noticed that “ethyl acetate” was an error could understand that the correct description was “ethyl acrylate”
It was known that ethyl acrylate was also abbreviated as EAC. Additionally, assuming that -OH group of Compound (3) nucleophilically attacks “EAC” to obtain Compound (4), the candidates of “EAC” were limited to (i) ethyl propionate or (ii) ethyl acrylate. It was understood that the volume and the number of moles of ethyl acrylate corresponded to “804 ml, 7.28 mol”. Accordingly, it was natural for those skilled in the art to notice that “EAC (ethyl acetate, 804 ml, 7.28 mol)” was an error in the description and understand that it should have read as “EAC (ethyl acrylate, 804 ml, 7.28 mol)”.
Conclusively, the IPHC upheld the Formosa’s appeal to accept the correction, and cancelled the JPO’s decision.
An appeal to the Supreme Court was NOT filed against this decision, and thus the decision is final and binding.
K&P’s Comments As seen from the above decision, a correction of error in the description after the grant is acceptable only when such an error is “clear” and those skilled in the art “naturally” deems that the error should have read as the corrected description, and the level of burden of proof thereof is very high. Accordingly, it is important to set up ample evidences proving beyond a reasonable doubt when filing a correction trial after the grant.
In May 2017, the IPHC handed down 15 decisions including the above case on patent, and overturned the previous decisions in 5 cases.
In May 2017, the IPHC handed down 2 decisions on trademark, and maintained all of the previous decisions.
In May 2017, the IPHC handed down 4 decisions on industrial design, and maintained all of the previous decisions.
Additionally, we have now confirmed that an appeal to the Supreme Court against the decisions on 2015 (Gyo-Ke) 10054 (Towa Pharmaceutical Co., Ltd. v. Merck Sharp & Dohme Corp.), which we introduced in the K&P’s IPHC Decision Report in March, 2016, was dismissed on June 1, 2017.



