- KAWAGUTI & PARTNERS Home>
- News Letter>
- August, 2020
K&P’sIntellectual Property High Court Decision Report in 2020
August, 2020
Updated 12 NOV 2020
1. How should Sameness of Invention in Art. 29bis of Patent Law be Examined?
AY Pharmaceuticals Co., Ltd. v. Otsuka Pharmaceutical Factory, Inc.; Case No. 2019 (Gyo-Ke) 10155 (Decision rendered on August 26, 2020)
The Patentee, Otsuka Pharmaceutical Factory (OPF), obtained a patent relating to an infusion solution preparation in 2008. Against the OPF’s patent, AY Pharmaceuticals (AYP) filed an invalidation trial with the JPO in 2018. During the trial proceedings, OPF demanded a correction of claims. The JPO admitted the correction, and dismissed the AYP’s demand in 2019. AYP filed an appeal against the JPO's decision to the IPHC in 2019.
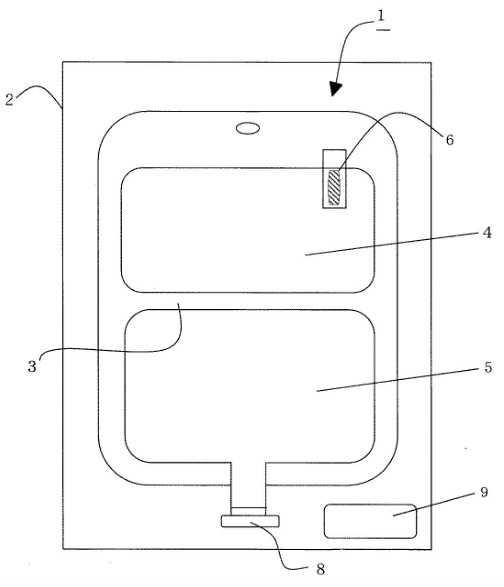
The corrected Claim 1 of the OPF’s patent at issue claims as follows:
An infusion solution preparation housed in an infusion solution container (1) having a plurality of chambers divided with partition means (3) capable of being communicated by pushing from the outside,
wherein a solution containing at least one compound selected from the group consisting of a sulfur-containing amino acid and a sulfite is filled in one chamber (5), and a trace metal element-storing container (6) in which a solution containing at least one trace metal element selected from the group consisting of Fe, Mn and Cu is stored in another chamber (4) is housed,
wherein the trace metal element-storing container (6) is a bag made of a thermoplastic resin film,
wherein the solution is an amino-acid infusion solution containing acetylcysteine, wherein the infusion solution container (1) is housed in a gas barrier outer bag (2), and
wherein oxygen in the outer bag (2) is removed.
One typical embodiment of the claimed preparation is as follows:
The figures in the prior application 1 to which the IPHC mainly refers in this decision is as follows.
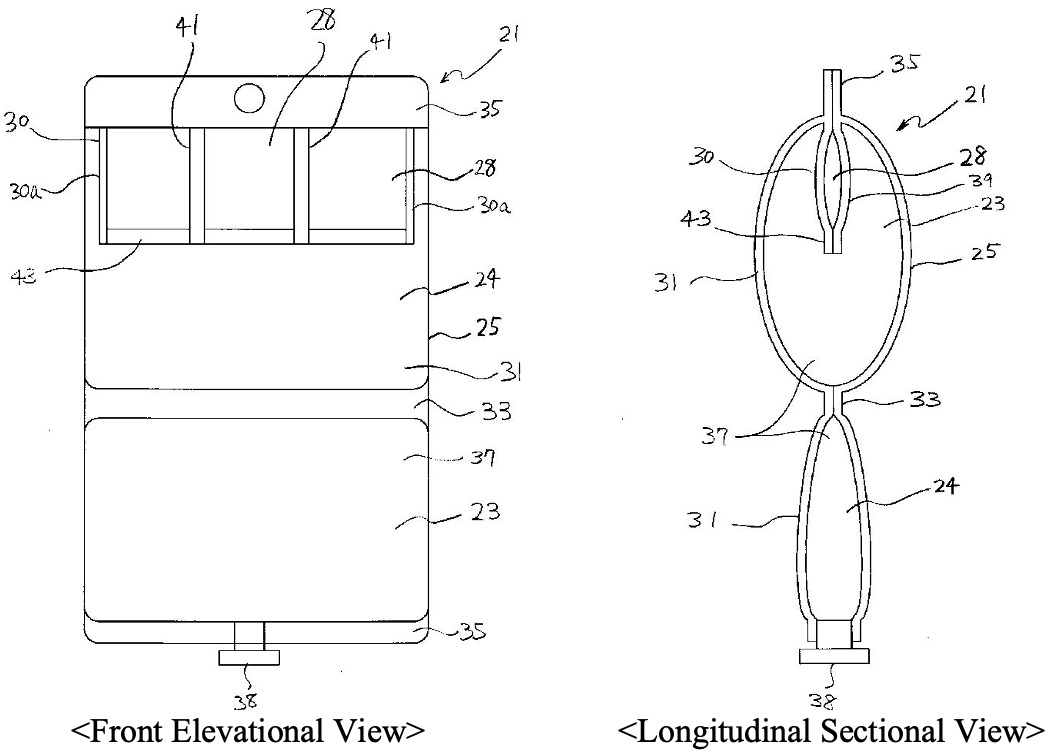
One of the main issues in this case related to how the sameness of an invention in Art. 29bis of the Japanese Patent Law, which corresponds to EPC 54 (3), should be examined. The IPHC answered to the issue as follows:
The IPHC reviewed the claimed preparation at issue and the disclosure of the prior application 1 in detail, and found three differences between them:
(i) that, in the former preparation, a trace metal element is stored in a trace metal element-storing container (6) which is housed in a different chamber from the chamber in which a solution containing at least one compound selected from the group consisting of a sulfur-containing amino acid and a sulfite is filled, where the trace metal element-storing container (6) is specified as being made of a film,
while in the latter disclosure, it is not specified where a trace metal element is stored, though plurality of divided chambers (storing containers) are housed in a different storing chamber from the storing chamber in which a solution containing an amino acid is filled, and it is unknown whether the plurality of divided chambers (storing containers) are made of a film;
(ii) that, in the former preparation, a solution contained in one chamber is an amino-acid infusion solution containing acetylcysteine, and the trace metal element-storing container (6) in which a solution containing at least one trace metal element selected from the group consisting of Fe, Mn and Cu is stored in the other chamber (4) is housed,
while in the latter disclosure, a solution containing an amino acid or an amino acid and an electrolyte is stored in one storing chamber, and at least two types of vitamins are separately stored in the plurality of divided chambers (storing containers) which are housed in the other chamber so that at least one vitamin is isolated from the other vitamins, and a part of the other vitamins may be stored in the storing chamber; and
(iii) that, in the former preparation, an infusion solution container (1) is housed in a gas barrier outer bag (2), and oxygen in the outer bag (2) is removed,
while in the latter disclosure, such limitations are not specified.
Regarding the differences (i) and (ii), the IPHC acknowledged (a) that the prior application 1 has no disclosure or suggestion on the basic technical concept of the claimed invention at issue, that is, a solution containing a trace metal element is stabilized by storing a solution containing a sulfur-containing compound in one chamber and housing the trace metal element-storing container (6) in the other chamber; (b) that it is optional in the prior application 1 whether cysteine or a salt, ester or N-acylated compound thereof is contained in the amino-acid infusion solution; and (c) further that there are plurality of options on where a trace metal element is located, and thus the location is not specified in the prior application 1.
On the basis of the above acknowledgement, the IPHC determined that it cannot be recognized that those skilled in the art understand the whole technical concept that cysteine or a salt, ester or N-acylated compound thereof, which corresponds to “acetylcysteine” specified in the claimed preparation at issue, is stored in the storing chamber 23 in the above figure and that a trace metal element is stored in the divided chamber 28 in the above figure; and thus that the differences (i) and (ii) are substantial differences.
Conclusively, the IPHC dismissed the AYP’s appeal and upheld the JPO’s decision.
An appeal to the Supreme Court was filed against this decision, and thus the decision is NOT made final and binding.
K&P’s Comments
As shown above, when examining the sameness of an invention in Art. 29bis of the Japanese Patent Law, the IPHC considered not only whether all the elements of the invention at issue are described or not in the prior application, but also whether the prior application discloses them so that the basic concept of the invention at issue can be acknowledged as a whole.
In other words, in order to apply the provision of Art. 29bis to deny patentability of the invention at issue, it is not sufficient to separately describe each of the elements of the invention at issue in any part of the prior application, but it is required that those skilled in the art can recognize the basic concept of the invention at issue as a whole from the disclosure of the prior application, which affirms the JPO’s practice.
In August, 2020, the IPHC handed down 9 decisions including the above case on patent, and overturned the previous decision in 1 case.
In August, 2020, the IPHC handed down 3 decisions on trademark, and maintained all the previous decisions.
In August, 2020, the IPHC handed down no decision on industrial design.
The chief judge of the IPHC changed from Ms. Makiko TAKABE to Mr. Ichiro OTAKA in October, this year.



