- KAWAGUTI & PARTNERS Home>
- IP Laws & Practices>
- Overview of the Patent Term Extension in Japan
About Japanese Practices
Law & RulesOverview of the Patent Term Extension in Japan
1. What is Patent Term Extension?Patents have a 20-year patent life from the date of the filing of the patent application [Art. 67 (1)].
The effective patent term is frequently less than 20 years in the field of pharmaceuticals and agrochemicals due to the requirements in the relevant laws that these products receive government approval before marketing.
In order to encourage inventions in these fields, the patent life is extended to compensate patent holders for marketing time lost while developing the product and awaiting government approval [Art. 67 (2)].
2. What is the maximum amount of time that a patent can be extended?The patent term can be extended by a maximum of 5 years.
A product would be eligible for patent extension even if the patent life of the product after approval has 14 or more years.
NOTE: Marketing time lost while developing a product and awaiting government approval can sometimes exceed 5 years. However, in view of the balance between the patent holder and the public, only a maximum of 5 years can be restored to the patent.
If the maximum 5 years were restored, however, the product’s total patent life would possibly exceed 14 years from the marketing approval. Even if this is the case, the patent holder may enjoy the maximum patent term extension of 5 years.
3. How is the length of patent term extension calculated?The extended patent term corresponds to the “period which the patented invention could not be worked” due to the requirements that pharmaceutical products receive government approval before marketing.
The period starts on the day when the clinical trial is started* or the day when the patent is registered**, whichever is later, and ends on the day just before the day when the regulatory approval is mailed to the applicant of the regulatory approval.
* the day when the IND is filed
**the day when the patent is granted
NOTE: The extended patent term corresponds to the “period which the patented invention could not be worked” due to the requirements that pharmaceutical products receive government approval before marketing, i.e. the regulatory review period. In the meaning of this section of the Patent Law, the period starts on the day when the clinical trial is started or the day when the patent is registered, whichever is later, and ends on the day just before the day when the regulatory approval is mailed to the applicant of the regulatory approval.
In the above, the day when the IND is filed is deemed as the day when the clinical trial is started. As to the day when the patent is registered, patents are granted and registered with the patent office upon payment of the registration fee. The day when the patent is registered therefore corresponds to the day when the patent is granted. Thereafter, a patent publication is issued but the issue date does not affect the above calculation.
The day when the regulatory approval is mailed is the day when the regulatory approval actually reaches the applicant for the marketing application.
Examples of the calculation
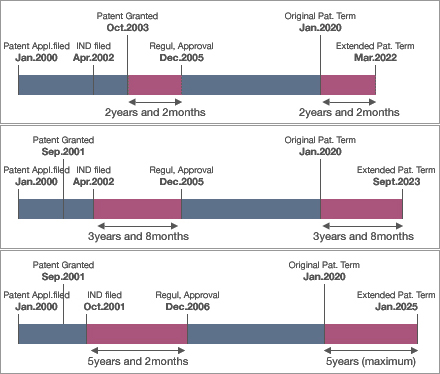
NOTE: The above shows examples of calculation. In the first case, filing of the IND is followed by the patent registration. Therefore, the extendable patent term corresponds to the period between the patent grant and the receiving of the regulatory approval.
In the second case, the period starts on the day the IND is filed because the patent grant is well before the filing date of the IND.
The third case shows that the maximum extension is 5 years.
AgrochemicalsThe period starts on the day when the field trial by an authorized agency* is started or the day when the patent is registered, whichever is later, and ends on the day just before the day when the regulatory approval is mailed to the applicant of the regulatory approval.
* The applicant for regulatory approval is required to submit a data package (toxicity, efficacy and etc.) obtained from the authorized field-trial agency.
NOTE: In the field of agrochemicals, the regulatory review period is composed of a field trial phase and an approval phase. The applicant for regulatory approval for agrochemical products is required to submit a data package (toxicity, efficacy and etc.) obtained from an authorized field-trial agency in Japan. The field trial phase starts on the day when the trial by the authorized agency is initiated.